TEMPLE, Texas — The U.S. Food and Drug Administration fully approved the first COVID-19 vaccine on Monday. Pfizer-BioNTech COVID-19 Vaccine, which will be marketed as Comirnaty, is now available for people over 16 without emergency use authorization (EUA).
Health officials like Department of State Health Services Senior Scientific Advisor Saroj Rai hope it will get more people on board.
"Now we do have a vaccine that is fully licensed, has gone though the standard review process that our regulatory process the FDA uses," Rai said.
Rai told 6 News the Pfizer vaccine has still been proven to be 91 percent effective in keeping people from being hospitalized by COVID-19, even with the new Delta variant on the rise.
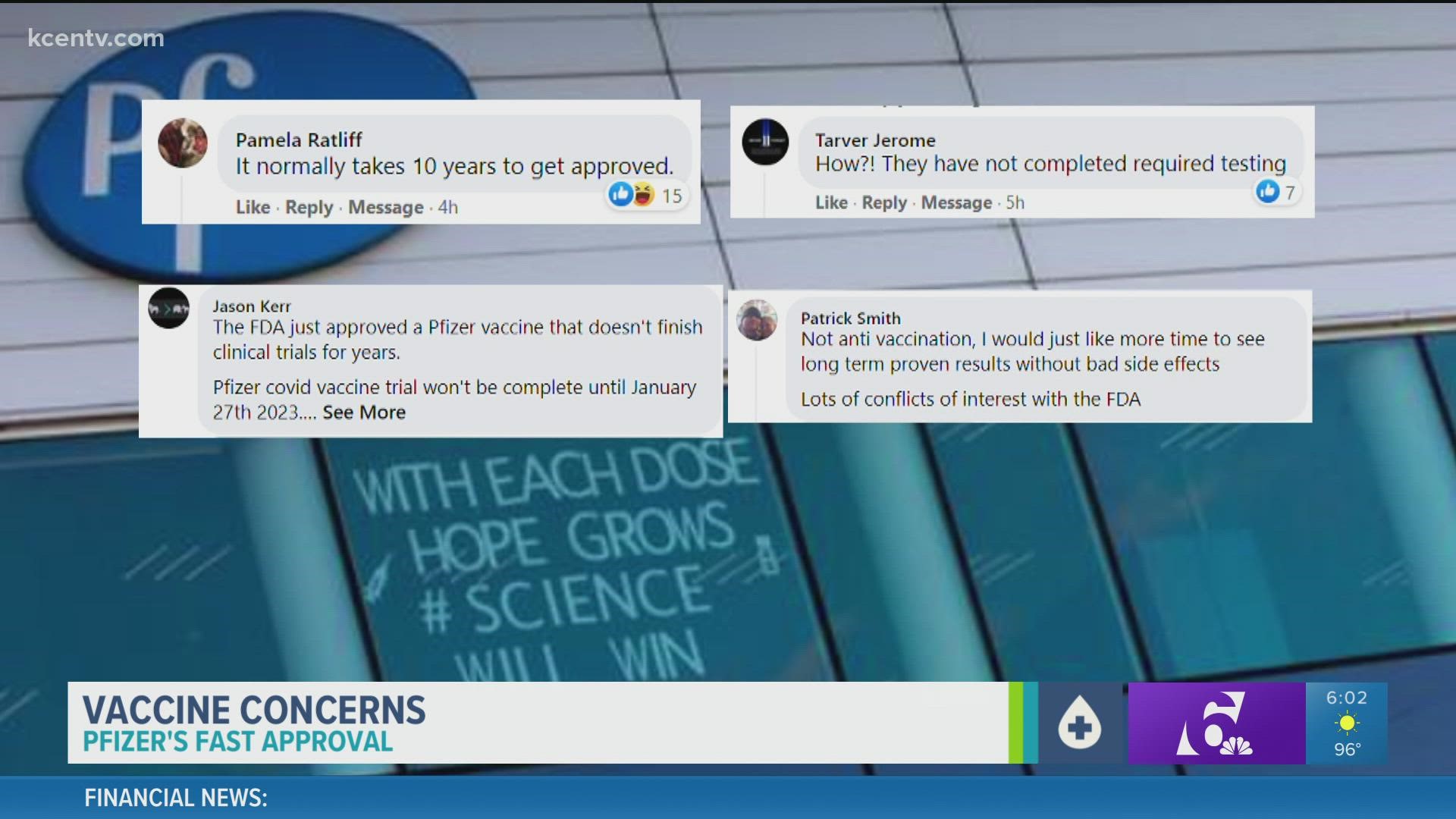
At the same time, the community response for 6 News on Facebook had plenty of posts doubting everything was fully studied. Rai walked 6 News though the regulatory process on Monday.
While the Pfizer vaccine was approved under emergency use in December of 2020, Rai said the first studies started all the way back in March of 2020. The first, second, and third phases of testing each started before the previous ones finished, as can be seen on this Johns Hopkins University of Medicine infographic. Manufacturing occurred at the same time. Under normal conditions, all these steps would happen one at a time.
Rai said that provided enough time for phase 3 trials to be completed with 6 months of follow-up afterwards. Rai also told 6 News that follow-up needed to continue after those 6 months even after approval was given.
"Part of the approval process is the FDA requires manufacturers to follow individuals for a longer period of time and do the ongoing safety monitoring. That is a requirement for the company as part of getting the full license," Rai said.
During that 6 month review, Rai said there have been a side effect warning added to both Pfizer and Moderna for a risk of Myocarditis, which is an inflammation of the heart tissue.
According to the CDC, there have been more than a thousand reports to the Vaccine Adverse Event Reporting System (VAERS) of cases of inflammation of the heart—called myocarditis and pericarditis—happening after mRNA COVID-19 vaccination.
In comparison, more than 177 million people have received at least one dose of COVID-19 vaccine in the United States.
Rai said the vaccine remains the most effective defense against COVID-19 and urges anyone who has not been vaccinated to get the shot right away.